Multiplatform
AI Ecosystem
for Drug Discovery
An integrated AI ecosystem designed to support early discovery decisions, strengthen rational therapeutic design, and provide clinically meaningful program translation.
Biology determines the strategy.
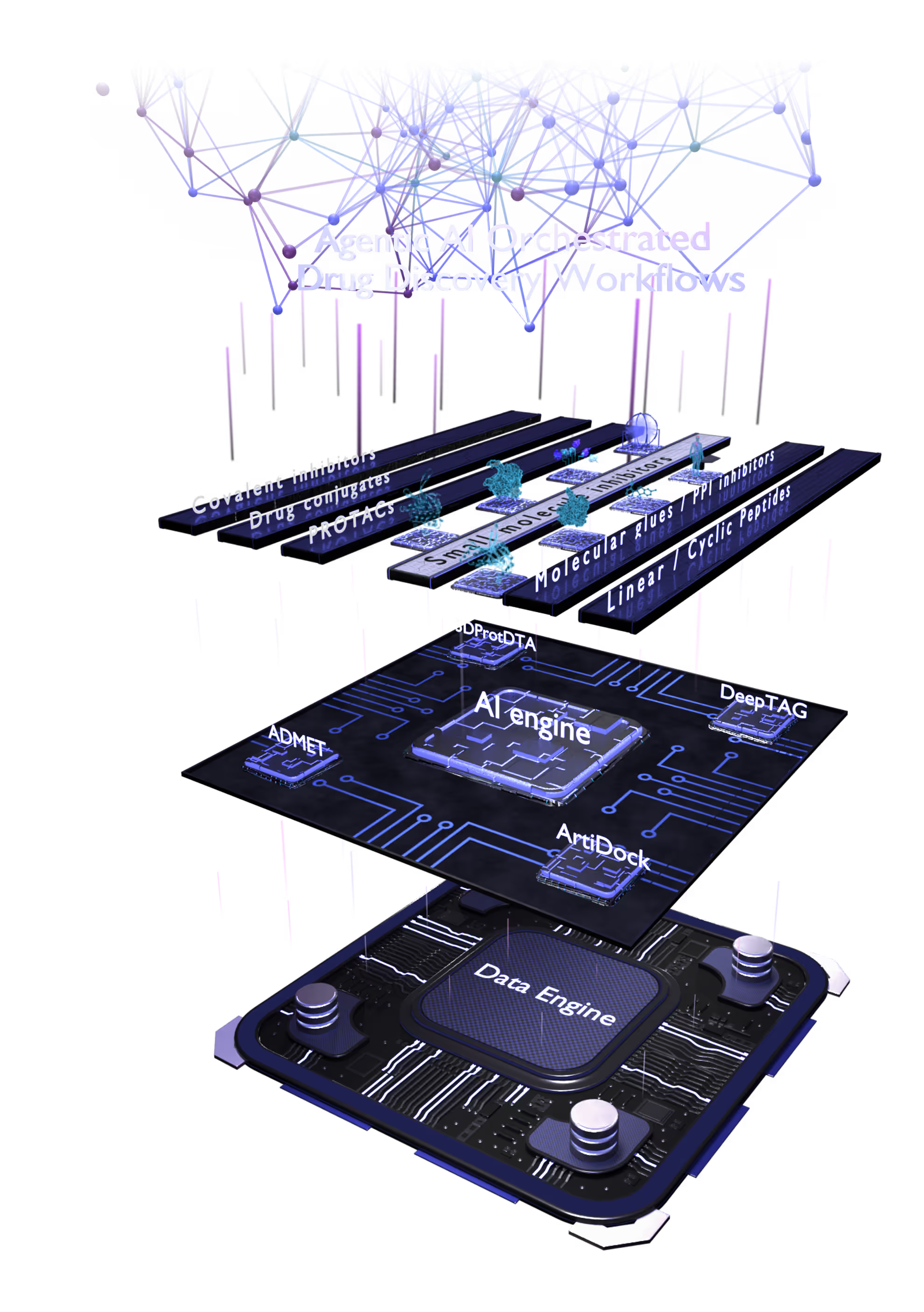
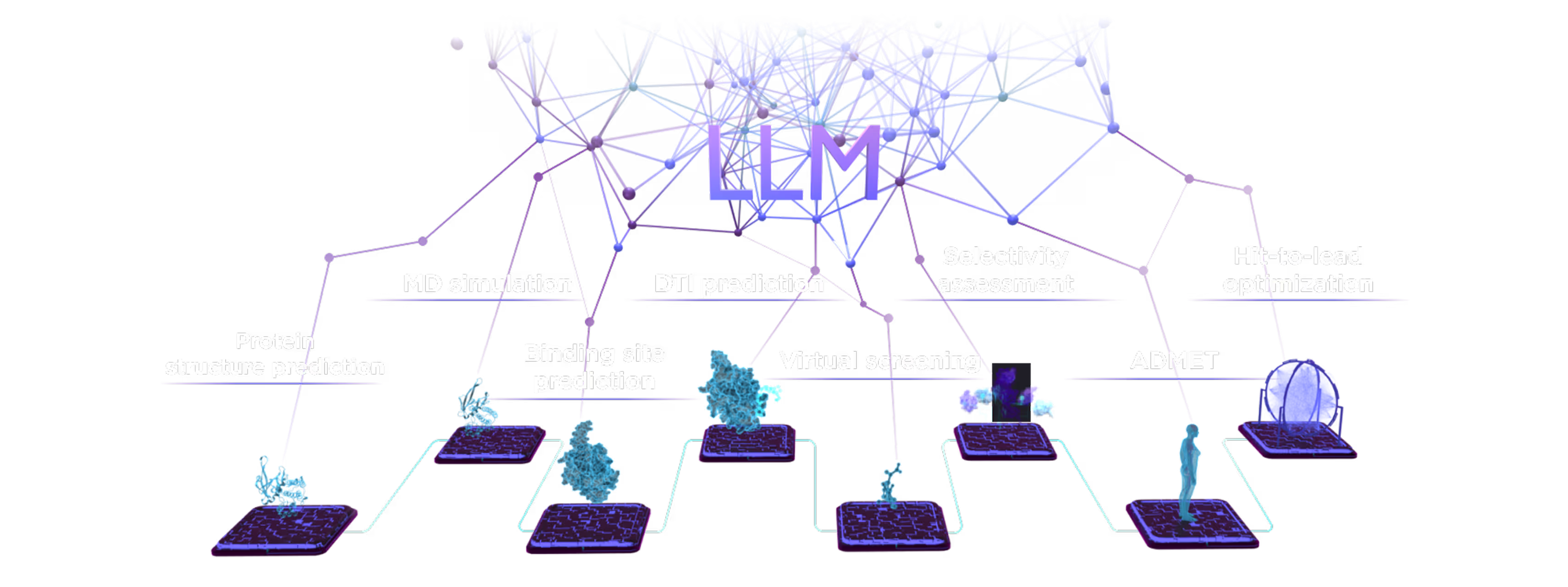
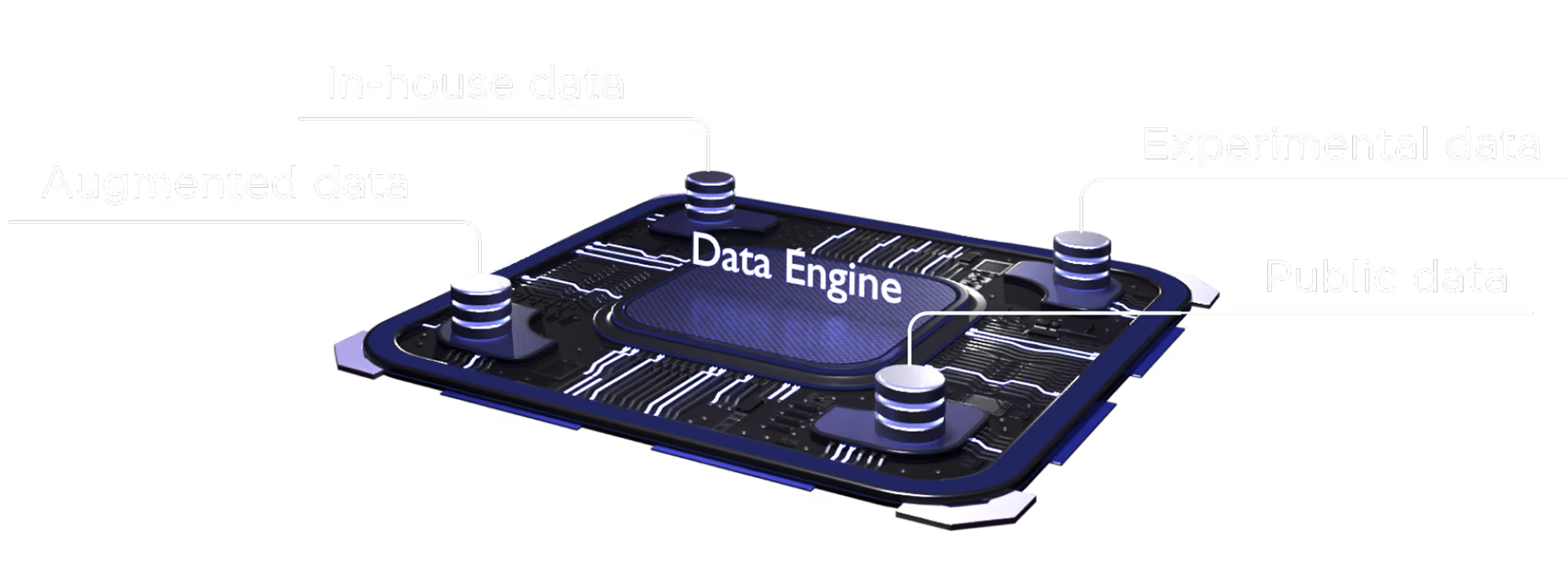
The multiplatfrom ecosystem delivers an objective, modality-agnostic therapeutic solution.
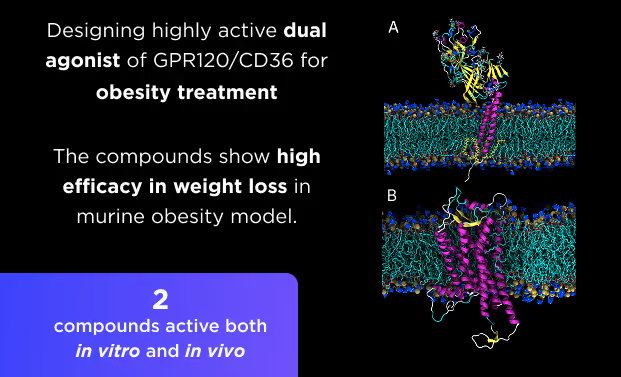
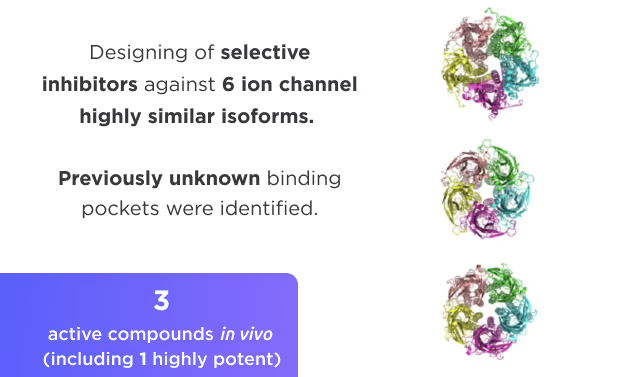
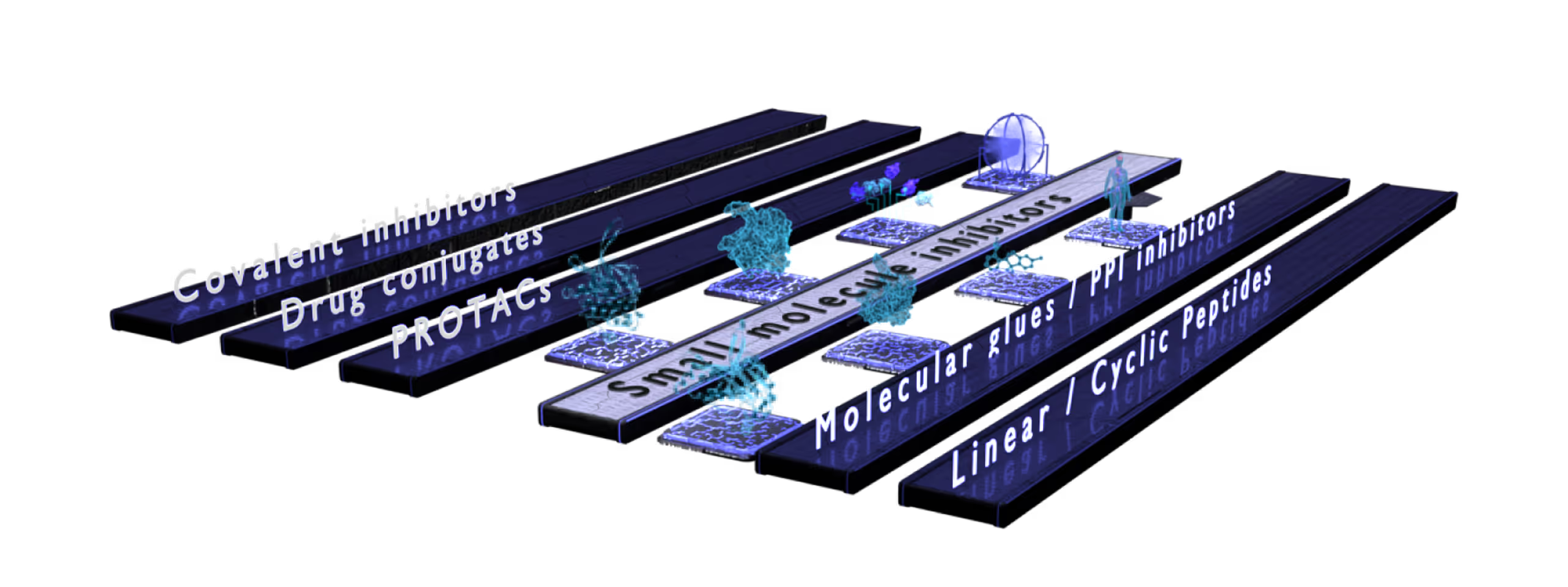
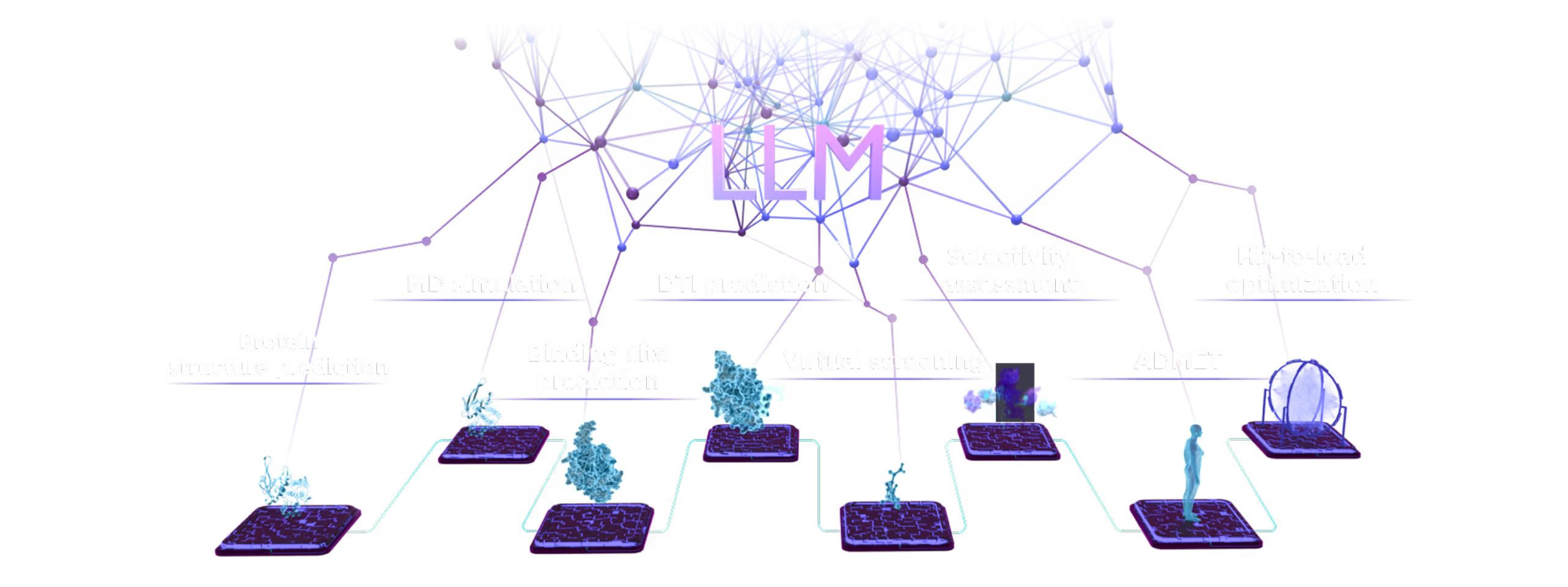
Small Molecules Platform
- AI drug discovery workflows for different target classes: kinases, GPCRs, ion channels, enzymes, etc.
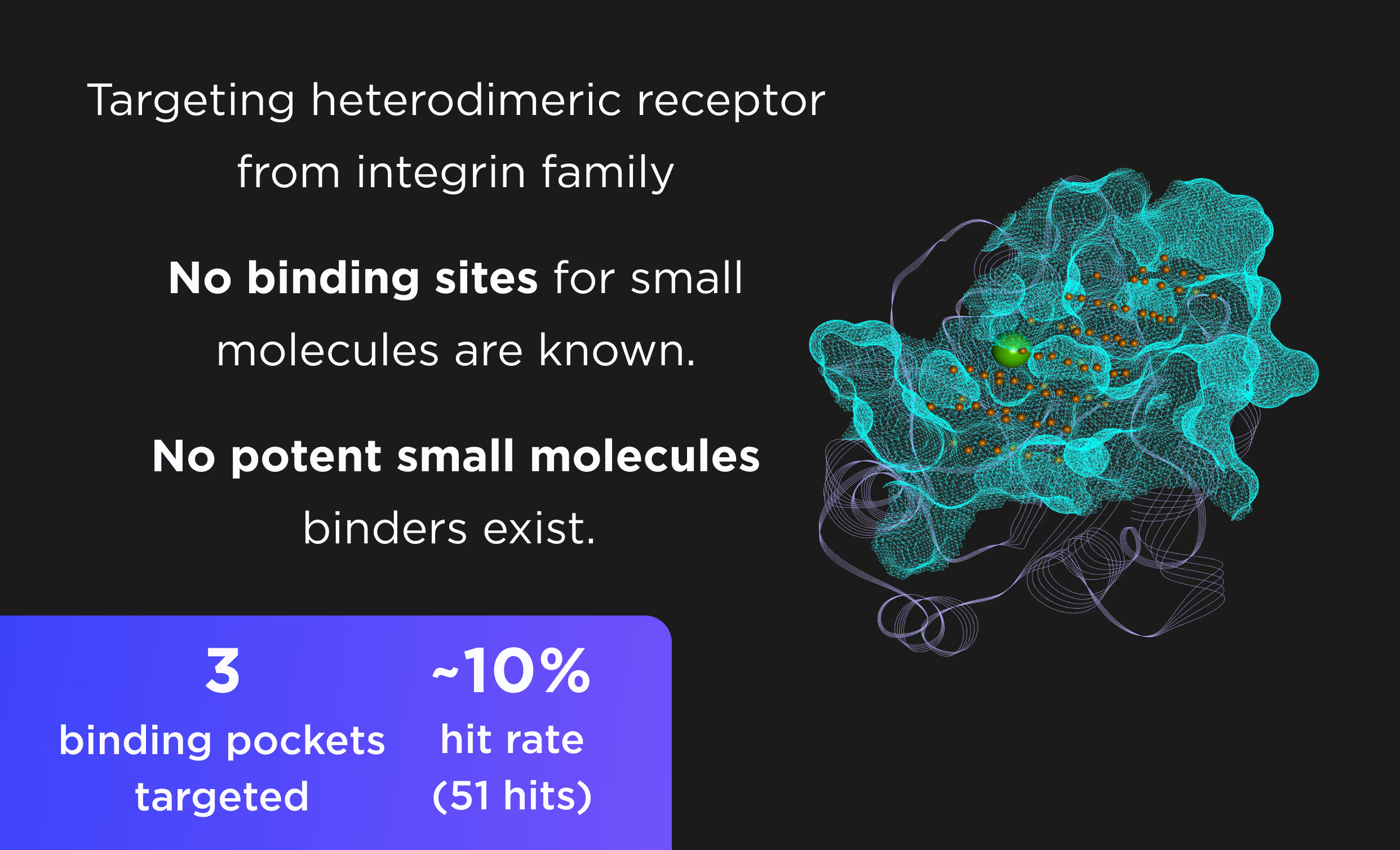
- AI pocket identification and targeting including allosteric, hidden, transient, and cryptic pockets.
- Automated SAR analysis with a hybrid intelligence approach; AI binding mode identification.
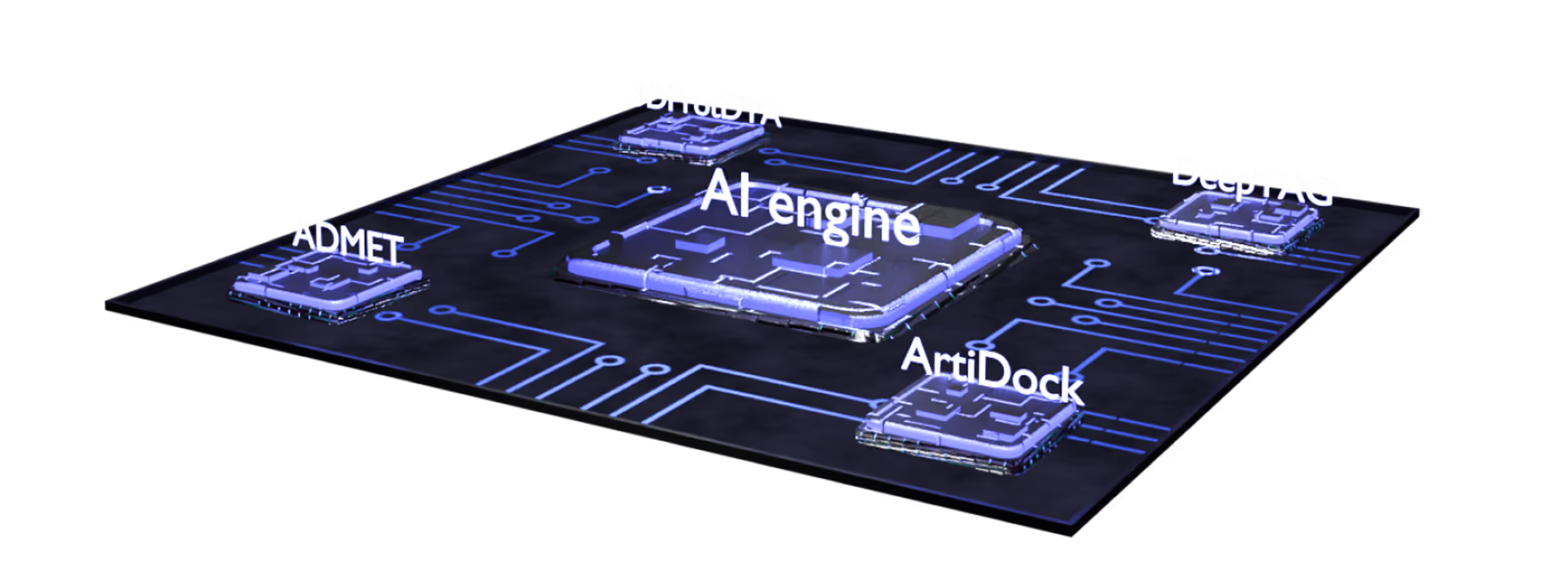
- Multiparametric virtual screening and lead optimization with QuorumMap.
- Selectivity optimization against highly similar isoforms and mutants.
- The largest set of ADMET endpoints for prediction and optimization with ADMETiQ.
Peptide Platform
- De novo binder design based on endogenous ligands, PPI partners, or display hits.
- Library of 10K+ non-canonical amino acids, non-peptide blocks, and linkage chemistry for unlimited diversity.
- Multiparametric optimization of peptide activity, permeability, and oral availability.
- Peptide-to-small-molecule workflow to develop compounds with superior ADMET properties.
- Scaffold-agnostic system that supports peptides of any topology and secondary structure.
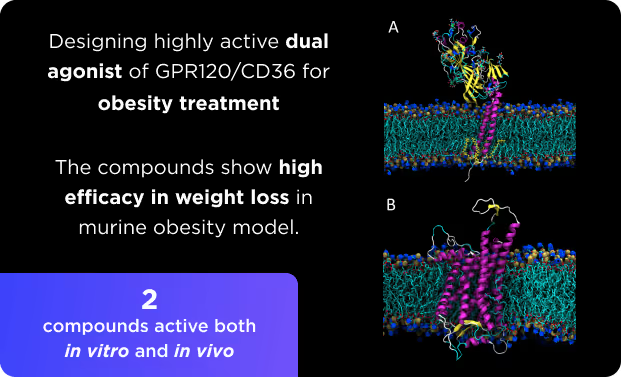
Proximity Inducers Platform
- Designing various proximity inducers, such as degraders and molecular glues and PPI disruptors.
- Enabling antibody-based therapeutics, including bispecifics, immune cell engagers, and antibody-guided scaffolds.
- Predicting PPI structures and ternary complexes without known structures and homology templates.
- Targeting both natural and induced PPIs, including transient and membrane interactions.
- Developing diverse modalities such as small molecules, peptides, antibody-derived formats, or drug conjugates.


.avif)
.avif)